

Volunteers ranging in age from 50 to 70 years old were randomly assigned to receive andexanet alfa or placebo in a 3:1 ratio (ANNEXA-A) or a 2:1 ratio (ANNEXA-R). The objective of the two parallel, randomized, double-blind, placebo-controlled trials was to determine the efficacy and safety of andexanet alfa for the reversal of anticoagulation effects from apixaban and rivaroxaban in healthy older volunteers. On May 03, 2018, the FDA approved andexanet alfa (Andexxa ®), the first and only specific antidote for anticoagulation reversal in patients treated with rivaroxaban or apixaban. The 4-F PCC proved effective for several hundred patients with DOAC-related bleeding events, but the need for a specific reversal agent remained. 6 However, although the agent showed correction of coagulation laboratory parameters (e.g., coagulation tests and thrombin generation), it was not consistent across all studies.
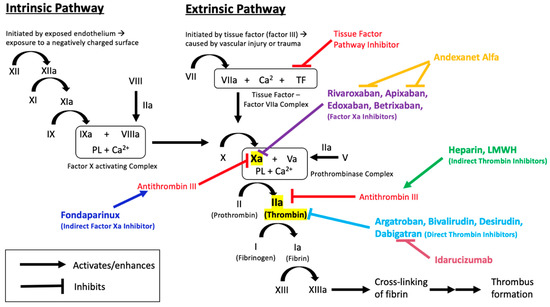
In 2017, the American College of Cardiology released a consensus decision pathway to manage DOAC-induced bleeding: Patients taking rivaroxaban and apixaban who experienced major bleeding events could receive a four-factor prothrombin complex concentrate (4-F PCC)––Kcentra (prothrombin complex concentrate ). 5 However, factor Xa inhibitors had no reversal agent at that time. It is a monoclonal antibody that binds dabigatran and its metabolite, neutralizing their anticoagulant effect. Idarucizumab (Praxbind) was the first reversal agent approved in 2015 for the direct thrombin inhibitor dabigatran (Pradaxa). Warfarin overdoses, on the other hand, are mitigated with vitamin K and clotting factor administration. 3 Although they comprise a promising group of medications, the DOACs initially had no reversal agents.

4įrom 2009 to 2014, DOAC treatment visits in the United States exceeded a million patients per quarter, a figure that is similar to the number of visits by warfarin patients. Most importantly, they have obviated the need for frequent testing of the international normalized ratio (INR) as is required with patients taking VKAs. 3 More recently, direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban, edoxaban, and betrixaban, have made their way onto the market and have shown comparable or greater efficacy, a desirable safety profile, and relatively simple dosing regimens compared to the VKAs. 2 For decades, vitamin K antagonists (VKAs) have been the traditional and only oral anticoagulant option but using them can be challenging owing to nuances in monitoring, dosing, and drug interactions. Atrial fibrillation is the most common type of arrhythmia, affecting approximately 2.7 to 6.1 million people in the United States, 1 and VTE affects about another 900,000 people each year. Oral anticoagulants are the medication of choice for the prevention of stroke in atrial fibrillation (AF) and the treatment of venous thromboembolism (VTE).


 0 kommentar(er)
0 kommentar(er)
